- What is Conductor, insulator & semiconductor?
- What is Intrinsic Semiconductor?
- What is Extrinsic Semiconductor?
- Crystal Structure & Behavior of semiconductor
- Comparative Analysis of Energy Level Diagrams
- Construction of PN junction diode
- How to find out the anode & cathode of a diode using a multimeter?
- What is Forward & Reverse Biasing of PN Junction?
- Rectifier Circuits: HWR, FWR & BR
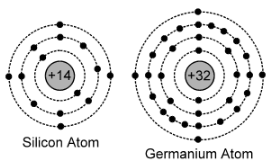
The pure crystal of semiconductor (Si or Ge) is known as intrinsic semiconductor at room temperature. The Silicon atom has 14 electrons revolving around its nucleus. In first orbit it contains 2 electrons, in second orbit 8 electrons i.e. 4 electrons in last orbit, known as valence electrons.
Similarly in Germanium atom, there are 32 electrons, such that 2+8+18+4 = 32. So the last orbit contains 4 electrons, known as valence electrons.
The crystal structure of pure Silicon at absolute zero temperature is shown below.
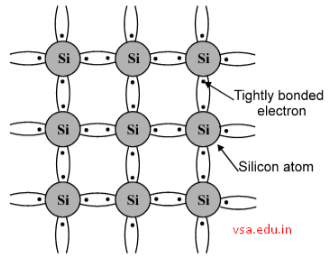
The valence electrons of each Si atom form covalent bonding with neighboring Si atoms. So there are no free electrons available to carry electric current. Thus at absolute zero temperature pure Si is a perfect insulator.
When external energy like heat, strong light or voltage is applied to crystal, the valence electrons acquire sufficient energy. So they break covalent bonds and jump into conduction band. The energy required to break conduction bands is about 1.12eV for Si and 0.75eV for Ge.
Holes in Semiconductor
In pure Si, when valence electrons break covalent bonds, they jump into conduction band. The electron which jumps out creates vacancy at its place. This vacancy is known as a hole.
A hole means actually nothing. But it can accept another electron from outside. Hence hole is assumed to have positive charge. When electron jumps out of valence band, it produces a hole. Thus a pair of one hole and one electron is produced, known as hole-electron pair.