- What is Conductor, insulator & semiconductor?
- What is Intrinsic Semiconductor?
- What is Extrinsic Semiconductor?
- Crystal Structure & Behavior of semiconductor
- Comparative Analysis of Energy Level Diagrams
- Construction of PN junction diode
- How to find out the anode & cathode of a diode using a multimeter?
- What is Forward & Reverse Biasing of PN Junction?
- Rectifier Circuits: HWR, FWR & BR
Some substances like Germanium, Silicon, Carbon, etc. are neither good conductors like copper nor insulators like glass. The resistivity of these materials lies in between conductors and insulators. Such substances are known as semiconductors.
Introduction
Semiconductor devices are very important parts in electronics. There are different types of semiconductor devices like diode, LED (Light Emitting Diode), bi-polar transistor, Integrated Circuit (IC), MOSFET (Metal Oxide Semiconductor Field Effect Transistor), FET, JFET (Junction Field Effect Transistor), SCR (Silicon Controlled Rectifier), etc. They are used in all types of systems for quality control and automation. Semiconductors are very fast in working, have low electrical consumption and long life.
The atomic structure and energy gap between valence band and conduction band plays important role in deciding the conductivity of the material. Thus conductivity depends on number of free electrons, to carry electric current.
Classification
Conductors: Copper, Gold, Silver, etc. contain large number of free electrons. Thus they are known as good conductor of electric current. There is no gap between valence band and conduction band (i.e. no forbidden gap), as shown below.
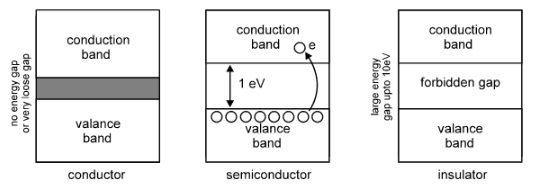
When voltage is applied, the free electrons start moving through the conductor. Thus in Copper, Gold, etc. electrons from valence band get extra energy from applied potential difference (PD) and they easily jump into conduction band. Such electrons become free from nuclear force.
Insulator: Rubber, plastic, ceramic, glass, etc. contain extremely few free electrons (practically zero). The forbidden gap between valence band and conduction band is very high (about 5eV-10eV). Thus in such materials, all electrons are tightly bounded with nuclear force.
Semiconductor: It is an artificial material. Its conductivity is between conductor and insulator, hence it is known as semiconductor. A semiconductor becomes a perfect insulator at absolute zero temperature. However, when temperature increases, the electrons in valence band absorb heat and they jump into conduction band. So its conductivity increases (i.e. resistance decreases) with temperature.
This property of semiconductor is known as Negative Temperature Coefficient of Resistance (NTC). Commonly used semiconductors are Silicon (Si-14) and Germanium (Ge-32). This NTC property of semiconductor is very useful in transducers like thermistors to measure the change in temperature. They are useful in medical and industrial instruments for temperature controlling systems.